

The advantages of ice
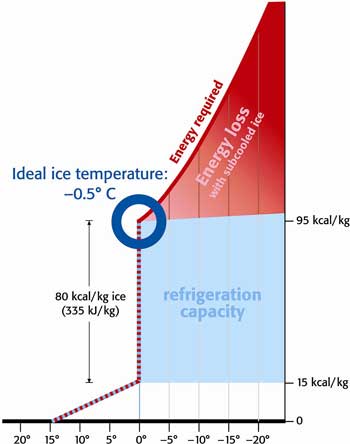
Ice packs a huge amount of cooling energy into a small volume, containing around 80KCals of latent cooling energy per kg. This latent cooling energy means that by melting from a kg of ice at 0°C into a litre of water at 0°C it extracts 80KCals of heat energy from a warm product. To put this in perspective, this is enough to reduce a kg (litre) of water from 90°C to 10°C. Even when a kg of ice has used all of this latent cooling energy by melting, you are still left with a litre of water at 0°C Chilled water has a cooling energy of 1Kcal/litre/°C, so as you can see, with its large 'battery' of latent cooling energy, ice is many times more efficient than chilled water. If you look at the diagram to the right, which shows the physics of freezing ice, you will see that ice is also efficient to produce. If you follow the dotted line, you can see that to turn water at 15°C into ice at 0°C requires 15kcals to cool the water to 0°C, then a further 80kcals turn this water into a kg of ice. As was stated above and can further be seen from the scale on the right hand side of the diagram, this ice now contains 80kcals of latent cooling energy, so we get back all of the electrical engergy we put in as cooling energy when we make ice at 0°C. In practice ice is always subcooled a little during the process of making it. half of 1°C in the case of chipIce and 7°C in the case of ScaleIce, but even in the case of ScaleIce, the subcooled wastes less than 5kcals, making all industrial ice types extremely efficient methods of cooling. |
 |
This has made ice very popular as a direct cooling medium in applications such as dough cooling in bakeries, where a certain amount of the chilled water in the recipe is swapped for ice, giving a big cooling effect, which translates into big gains in product yield and quality. Yet despite its efficiency, ice is a very gentle form of cooling, acting as its own thermostat. As soon as ice is put on or into a warm product, heat begins to flow out of the product and into the ice and begins to melt it. The heat carries on flowing until there is no difference in temperature between the two, so the product is cooled to the temperature of the ice, but no further. Once the product has cooled to the temperature of the ice, any further melting of ice that occurs is due to heat from external sources - the warm surrounding air, for example. So once the product is cool, the ice carries on keeping the product cool, by continuosly melting and sacrificing itself. When a product is surronded by ice, it is impossible for it to rise to a temperature warmer than the ice until all the ice has melted away. This means that ice on a product is proof positve that a product has not been above 0°C since being packed, making it very popular for packing perishable products during transport. Another positve side effect is that by melting into water, not only is the ice keeping the product cool, but is also keeping the product moist, unlike refrigerated air, which has a dehydrating effect. This is one of the main reasons why ice is popular for packing and displaying products such as fish, which become very unattractive if allowed to dry out. Another feature of ice types such as chipIce and nugget ice is that they are a very attractive white colour, so they are often used to display salads, fish, drinks etc in food service locations, restaurants and retail outlets. |
 |
Ziegra Ice Machines (UK) Ltd Unit 2, Phoenix Court, Hammond Avenue, Stockport, Cheshire, SK4 1PQ Tel:0844 8808055 Fax: 0161 480 7927 Email: ice@ziegra.co.uk |